Overview
PDS Biotech’s proprietary cationic, lipid-based nanoparticle platform technology called Versamune® is designed to harness the power of T cells to successfully recruit, train and arm T cells to execute a precise attack against the targeted disease.
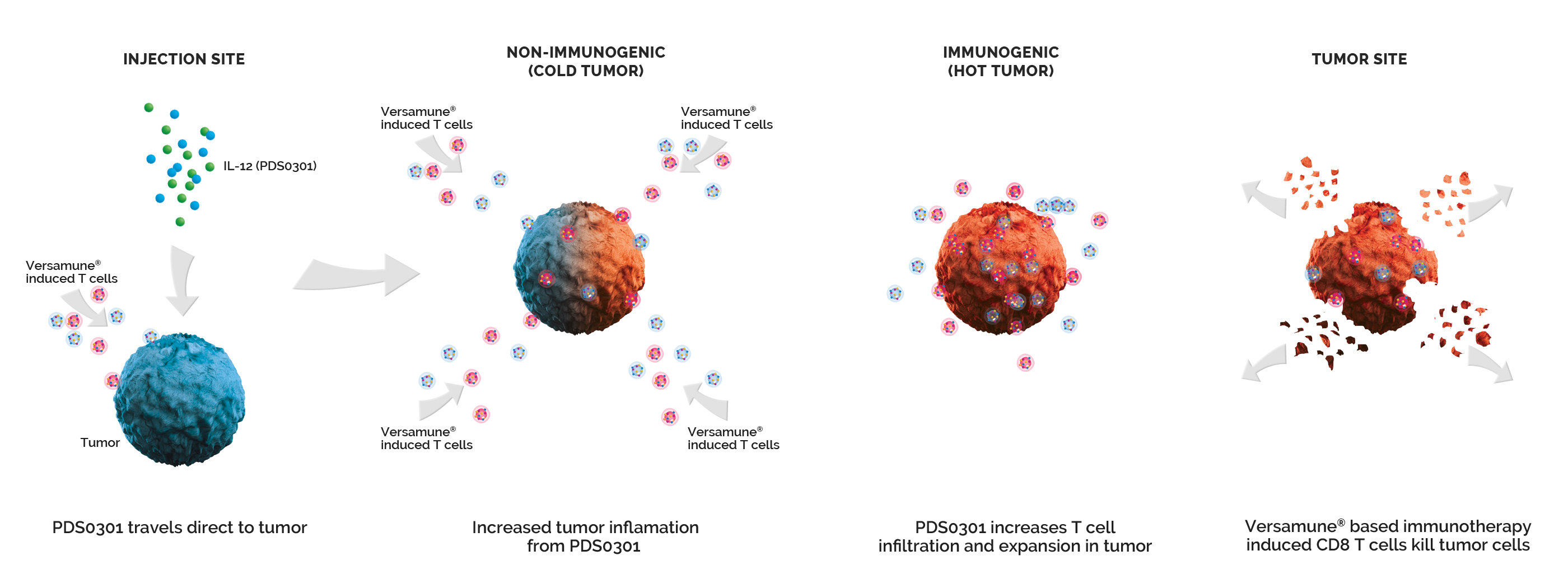
PDS Biotech’s fused IL-12 antibody drug conjugate (PDS01ADC) is designed using an antibody that binds to necrotic DNA that is found within tumors. The antibody therefore is able to specifically target and deliver the IL-12 into the tumor’s inner microenvironment to suppress the tumor’s ability to protect against T cells. The IL-12 also promotes T cell infiltration into the tumor and activation of T cells.
To date, based on the results of several ongoing clinical trials these technologies appear to safely promote tumor shrinkage and patient survival.
We are developing multiple immunotherapy products based on PDS01ADC and our Versamune® platform. We believe these products have the potential to save and improve the lives of patients worldwide:
About Versamune®
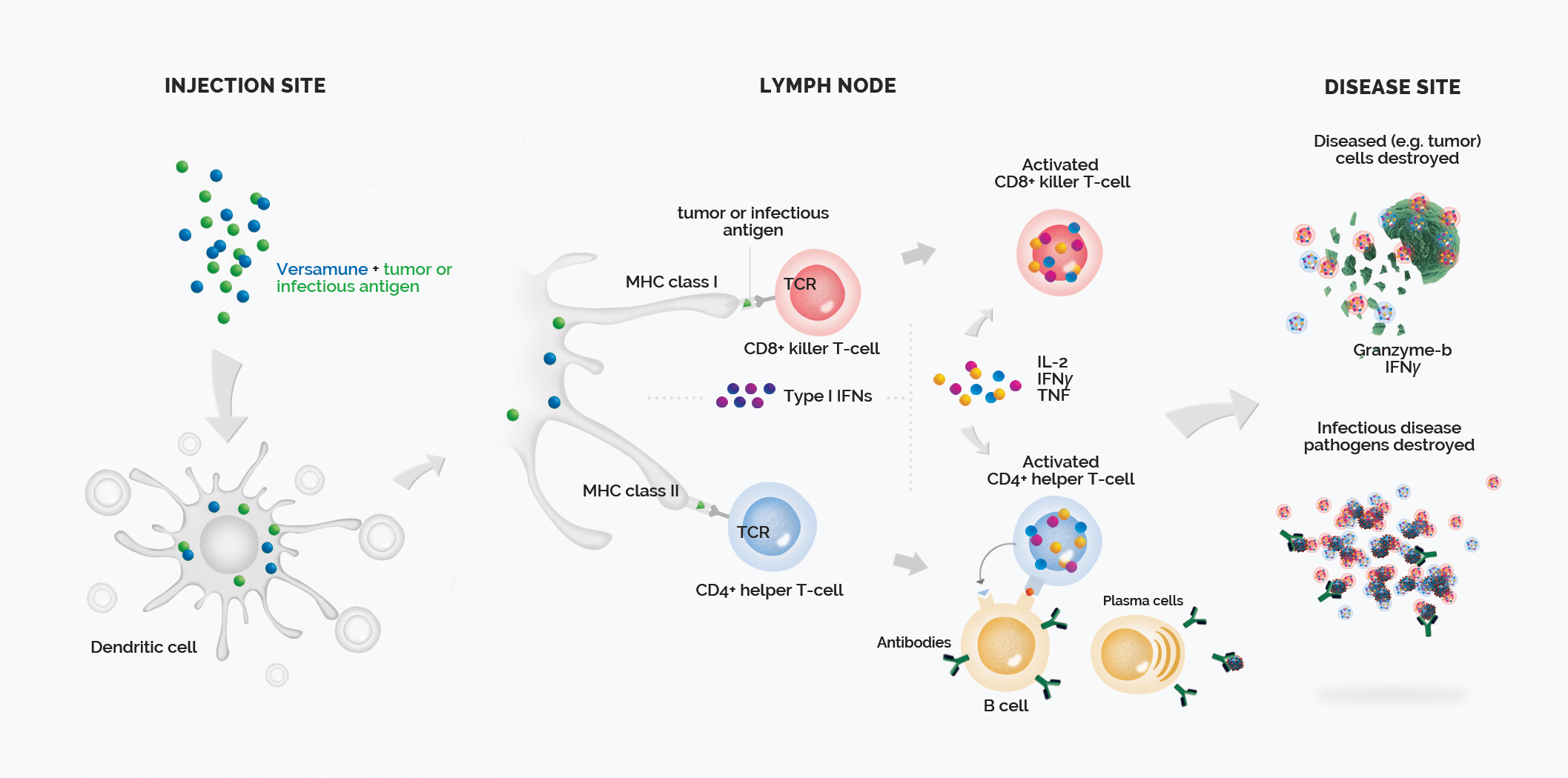
Versamune® is a novel investigational T cell activating platform which effectively stimulates a precise immune system response to a cancer-specific protein. Versamune® based immunotherapies promote a potent targeted T cell attack against cancers expressing the protein and are given by a simple subcutaneous injection and can be combined with standard of care treatments. Clinical data suggest Versamune® based immunotherapies demonstrate significant disease control by shrinking tumors, delaying disease progression and/or prolonging survival. Versamune® based immunotherapies have demonstrated minimal toxicity to date that may allow them to be safely combined with other treatments. Versamune® based investigational immunotherapies represent a transformative treatment approach for cancer patients and provide improved efficacy, safety and tolerability.
Versamune®, when combined with unique proteins or peptides that are specific to the cancer (antigens), is demonstrated to:
 Induce disease targeting T-cells, including a highly potent type of T-cell called a CD8+ or killer T-cell, that is effective in attacking cancer |
 Promote both a large quantity and high quality of tumor-specific killer and helper T-cells, tailored to infiltrate and attack cancer |
 Deliver a powerful immune response associated with regression of cancer |
 Deliver efficacy with minimal side effects – to date limited to transient injection site reactions without triggering more severe systemic side effects |
 Substantially improve the activity of other proven cancer-fighting technologies – e.g. checkpoint inhibiters and cytokine-based therapies – when administered in combination with our Versamune®-based therapies |
Mechanism of Action
The Versamune® platform is comprised of structure specific positively charged (cationic) lipids co-administered with custom-designed antigens specific to the types of cancer of interest. These combinations, delivered via simple subcutaneous injection, have proven to stimulate the activation of multiple critical immunologic pathways associated with effective T-cell induction and increased cancer-fighting activity.
Versamune® is based on the R-enantiomer of 1,2-dioleoyl-e-trimethyl-ammonium-propane (R-DOTAP), a cationic lipid. The lipid spontaneously assembles into nanoparticles in a water-based medium and is sized to mimic an artificial virus. This promotes efficient uptake by dendritic cells. Versamune® has been demonstrated to promote cross-presentation of the antigen to both CD4+ helper and CD8+ killer T-cells. Versamune® has also been proven to activate the Type I Interferon pathway, leading to superior recruitment and priming of highly potent, polyfunctional, T-cells.
Powerful Response
Versamune® has been shown to induce a significantly higher quantity and quality of highly potent (polyfunctional) killer T-cells, a specific sub-type of killer T-cell that is more powerful at attacking cancer.
By promoting the induction of the right type of killer T-cell, with strong potency and in the right quantity, Versamune® results in more effective cancer treatments when compared to other technologies. Versamune®-based products demonstrate impressive efficacy when used alone in specific types of cancer. However, when the powerful immune response of Versamune® is added to proven checkpoint inhibitor therapies, which help “unmask” cancer tumors, the two technologies have been demonstrated in early studies to work synergistically to attack and destroy cancer cells more effectively than either alone.
Safety
Versamune® offers a favorable safety profile:
- Side effects limited predominantly to transient injection site reactions
- No dose limiting side effects observed to date
- No long-term safety concerns
- Versamune® avoids potentially systemic toxicities associated with most other cancer fighting technologies, such as chemotherapies, CAR-T cells, and other immuno-oncology agents. The safety profile of Versamune®-based products offers great potential for combination with both proven and new cancer therapies.
Simple Administration
Versamune® is engineered for simplicity and ease of administration. Versamune® is delivered together with proprietary antigens tailored for specific cancers. Versamune®-based immunotherapies are injected subcutaneously.
Ease Of Manufacturing
Versamune®-based cancer immunotherapies are easily manufactured and highly stable.
Intellectual Property
PDS Biotech’s currently holds several patents in major markets, including US, Europe and Japan, providing multiple layers of technology and product protection through at least the mid-2030s.
White Paper
A Simplified Overview of How the Versamune® Platform Overcomes Key Limitations of Immuno-Oncology
DOWNLOADAbout Versamune® plus PDS01ADC
Versamune® plus PDS01ADC amplifies the power of Versamune® based immunotherapy with PDS01ADC. PDS01ADC is a novel investigational tumor-targeting IL-12 fused antibody drug conjugate that enhances the proliferation, potency and longevity of T cells in the tumor microenvironment. Together they work synergistically to promote a targeted T cell attack against recurrent/metastatic cancers. Versamune® plus PDS01ADC represents a transformative treatment approach for recurrent/metastatic cancer patients by potentially providing improved survival and anti-tumor efficacy with acceptable safety and tolerability.
Mechanism of Action
PDS01ADC (IL-12 Fused Antibody Drug Conjugate) Targets the Tumor’s internal microenvironment and suppresses the tumor’s defenses and also enhances T Cell Infiltration and Proliferation in the Tumor
About Infectimune®
Infectimune® is a novel investigational immune activating platform which generates broad and robust antibody and T cell responses that provide durable protection against infectious disease. Infectimune® based vaccines are given by intramuscular injection and generate robust and durable protection against infectious agents in preclinical studies. Infectimune® based vaccines have demonstrated safety in preclinical studies and appear to provide more robust and longer-lasting protection against infectious disease.
Infectimune® is formulated to activate the immune system to:
 Induce rapid and longer-lasting neutralizing antibody responses for improved protection against infectious pathogens |
 Induce memory T-cell responses to provide the immune system with long-term memory and sustained protection against infectious pathogens over an extended period of time exceeding traditional antibody-based protection. |
 Provide safe and effective vaccines that are well tolerated by healthy individuals |